Final answer:
The empirical formula of the compound is
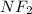 .
.
Step-by-step explanation:
To determine the empirical formula of the given compound, we need to find the smallest whole number ratio of nitrogen to fluorine. We can assume 100g of the compound to make the calculations easier.
Given that the compound contains 26.94g of nitrogen and 73.06g of fluorine, we can convert these masses into moles using their respective molar masses. The molar mass of nitrogen is 14.01 g/mol, and molar mass of the fluorine is 19.00 g/mol.
So, 26.94g of nitrogen is approximately equal to 1.92 moles, and 73.06g of fluorine is approximately equal to 3.85 moles. Dividing the moles of each element by the smallest number of moles, we get the empirical formula of
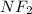 .
.