Answer:
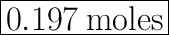
Step-by-step explanation:
The number of moles of ammonia can be found by using the formula:

where
m is the mass in grams
M is the molar mass in g/mol
n is the number of moles
From the question:
m = 3.360 g
M = 17.030 g/mol
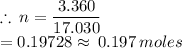
We have the final answer as;
0.197 moles