Answer:
If 7.9 moles of C₅H₁₂ reacts with excess O₂, 39.5 moles of CO₂ will be produced.
Step-by-step explanation:
The balanced reaction is:
C₅H₁₂ + 8 O₂ → 5 CO₂ + 6 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
- C₅H₁₂: 1 moles
- O₂: 8 moles
- CO₂: 5 moles
- H₂O: 6 moles
Then you can apply the following rule of three: if by stoichiometry 1 mole of C₅H₁₂ produces 5 moles of CO₂, then 7.9 moles of C₅H₁₂ will produce how many moles of CO₂?
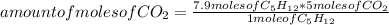
amount of moles of CO₂= 39.5 moles
If 7.9 moles of C₅H₁₂ reacts with excess O₂, 39.5 moles of CO₂ will be produced.