Answer:
1.389 moles
Step-by-step explanation:
We are given that
Given mass of ammonium=25.0 g
We have to find the number of moles in 25.0g of ammonium.
The chemical formula of ammonium
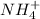
Molar mass of N=14, Molar mass of H=1
Molar mass of ammonium (NH4+)=14+4(4)=18 g/mol
We know that
Number of moles=Given mass/Molar mass
Using the formula
Number of moles of ammonium=
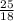 moles
moles
Number of moles of ammonium=1.389 moles
Hence, the number of moles in 25.0g of ammonium=1.389 moles