Final answer:
To calculate the quantity of heat that needs to be removed from the water and the aluminum can for their temperatures to fall to -5.0 °C, we can use the heat transfer equation. The quantity of heat that needs to be removed from the water is -16,800 J and the quantity of heat that needs to be removed from the aluminum can is -1,800 J. The total quantity of heat that needs to be removed is -18,600 J.
Step-by-step explanation:
To calculate the quantity of heat that needs to be removed from the water and the aluminum can for their temperatures to fall to -5.0 °C, we can use the heat transfer equation:
Q = mcΔT
For the water:
- Mass (m) = 200 g
- Specific heat capacity (c) = 4.20 J/(g °C)
- Change in temperature (ΔT) = (-5.0 °C) - (15 °C) = -20 °C
Substituting these values into the equation:
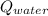 = (200 g)(4.20 J/(g °C))(-20 °C) = -16,800 J
= (200 g)(4.20 J/(g °C))(-20 °C) = -16,800 J
For the aluminum :
- Mass (m) = 100 g
- Specific heat capacity (c) = 0.90 J/(g °C)
- Change in temperature (ΔT) = (-5.0 °C) - (15 °C) = -20 °C
Substituting these values into the equation:
 = (100g)(0.90J/(g °C))(-20 °C) = -1,800 J
= (100g)(0.90J/(g °C))(-20 °C) = -1,800 J
The total quantity of heat that needs to be removed from the water and the can is the sum of
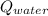 and
and
 :
:
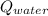 +
+
 = -16,800 J + (-1,800 J) = -18,600 J
= -16,800 J + (-1,800 J) = -18,600 J