The back work ratio is defined as the ratio of the compressor work to the turbine work. For an ideal Brayton cycle (where the compressor and turbine processes are isentropic), this is calculated as follows:
![\[ BWR = (W_c)/(W_t) \]](https://img.qammunity.org/2024/formulas/physics/high-school/hy44y87mak5l0grutx6lbtnn9e54ru9qh4.png)
Thermal efficiency for a Brayton cycle is defined as the net work done by the system divided by the heat input:
![\[ \eta_(th) = (W_(net))/(Q_(in)) \]](https://img.qammunity.org/2024/formulas/physics/high-school/my6av1ecy8l88rfrwxwqcorezidu9h4gez.png)
To determine the back work ratio and thermal efficiency for a system with a compressor efficiency of 80% and a turbine efficiency of 85%, we need to follow a step-by-step thermodynamic analysis. This kind of analysis is typically used in the context of a simple Brayton cycle, which is a common model for gas turbine engines.
Step 1: Understand the Brayton Cycle
The Brayton cycle consists of four processes:
1. Isentropic compression (in a compressor).
2. Constant pressure heat addition (in a combustion chamber).
3. Isentropic expansion (in a turbine).
4. Constant pressure heat rejection (in the exhaust).
Step 2: Assumptions
- The cycle operates in a steady flow.
- Changes in kinetic and potential energy are negligible.
- The working fluid behaves as an ideal gas.
Step 3: Define Given Efficiencies
- Compressor efficiency
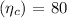
- Turbine efficiency
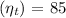
Step 4: Back Work Ratio (BWR)
The back work ratio is defined as the ratio of the compressor work to the turbine work. For an ideal Brayton cycle (where the compressor and turbine processes are isentropic), this is calculated as follows:
![\[ BWR = (W_c)/(W_t) \]](https://img.qammunity.org/2024/formulas/physics/high-school/hy44y87mak5l0grutx6lbtnn9e54ru9qh4.png)
However, with non-ideal conditions (real efficiencies), the work input and output are different. The actual work of the compressor
 can be adjusted for efficiency:
can be adjusted for efficiency:
![\[ W_c(actual) = (W_c(ideal))/(\eta_c) \]](https://img.qammunity.org/2024/formulas/physics/high-school/9v0w6iq49m4x12uai6ra7bj571bqyd8j4r.png)
![\[ W_t(actual) = W_t(ideal) * \eta_t \]](https://img.qammunity.org/2024/formulas/physics/high-school/aca1o0r6b5h7xkcf56fim4o89ygbqahd17.png)
The ideal work can be expressed in terms of specific heats and temperature differences (for an ideal gas), but without specific temperatures or pressure ratios, we can't calculate this exactly. Instead, we can express BWR in terms of efficiencies:
![\[ BWR = (W_c(actual))/(W_t(actual)) = (W_c(ideal) / \eta_c)/(W_t(ideal) * \eta_t) \]](https://img.qammunity.org/2024/formulas/physics/high-school/nh0vbknize4r3k62ldqa2qjv23aqgs24vo.png)
Since we don't have the exact values for
 we cannot calculate a numeric value for BWR without additional data.
we cannot calculate a numeric value for BWR without additional data.
Step 5: Thermal Efficiency
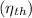
Thermal efficiency for a Brayton cycle is defined as the net work done by the system divided by the heat input:
![\[ \eta_(th) = (W_(net))/(Q_(in)) \]](https://img.qammunity.org/2024/formulas/physics/high-school/my6av1ecy8l88rfrwxwqcorezidu9h4gez.png)
Where
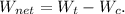 Again, without specific values for work and heat transfer, we can't compute a numeric value. However, we can express it in terms of given efficiencies:
Again, without specific values for work and heat transfer, we can't compute a numeric value. However, we can express it in terms of given efficiencies:
![\[ \eta_(th) = (W_t(actual) - W_c(actual))/(Q_(in)) \]](https://img.qammunity.org/2024/formulas/physics/high-school/fzhfs8rftlxzg2akqxin3tsyxtml31gxeh.png)