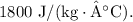The heat capacity C of the piece of wood is
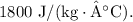
To calculate the heat capacity of a piece of wood, follow these steps:
1. Determine the mass of the wood:
Given:
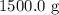 . To use the formula for heat capacity, we need to convert this mass into kilograms because the specific heat capacity is typically expressed in
. To use the formula for heat capacity, we need to convert this mass into kilograms because the specific heat capacity is typically expressed in
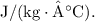
![\[ 1500.0 \text{ g} = 1.500 \text{ kg} \]](https://img.qammunity.org/2024/formulas/physics/high-school/y9ralhmv3m2n4nkx2y37fkzp5a4akqn8j5.png)
2. Calculate the change in temperature (
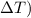 :
:
Change in temperature:
![\( \Delta T = T_{\text{final}} - T_{\text{initial}} \) \[ \Delta T = 57°C - 32°C = 25°C \]](https://img.qammunity.org/2024/formulas/physics/high-school/w6jryaiaqr91bwnyx5qb3izzomco4dd7p9.png)
3. Calculate the heat absorbed ( Q ):
Given:
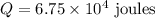
4. Apply the formula for heat capacity( C ):
The formula to calculate the specific heat capacity ( c ) is:
![\[ c = (Q)/(m \cdot \Delta T) \]](https://img.qammunity.org/2024/formulas/physics/high-school/b2a4v3u101tjxbd4werclwn072u2mlcqkt.png)
where Q is the heat absorbed, m is the mass in kilograms, and
 is the change in temperature.
is the change in temperature.
5. Solve for the specific heat capacity:
Plug in the known values and solve for c :
![\[ c = \frac{6.75 * 10^4 \text{ J}}{1.500 \text{ kg} \cdot 25°C} \]](https://img.qammunity.org/2024/formulas/physics/high-school/4ovde6vjojujbjni80wjpk30tai34w3nmw.png)
When we perform the calculation, we find that the specific heat capacity of the wood is