Answer:

Step-by-step explanation:
The molarity of the solution can be found by using the formula:

c is the concentration in M , mol/dm³ or mol/L
v is the volume in L or dm³
m is the mass in grams
M is the molar mass in g/mol
From the question;
mass = 5 g
Molar mass (M) of KCl = 39 + 36.5 = 75.5 g/mol
Since the volume is in mL it has to be converted to litres.
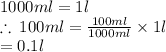
v = 0.1 L
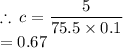
We have the final answer as;
0.67 M