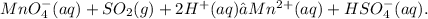Final answer:
To balance the given equation Mno⁴⁻ + SO² gives Mn² + HSO⁴⁻ in acidic medium, you need to follow the steps of the half-reaction method. Using these steps, the balanced equation is
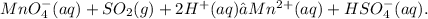
Step-by-step explanation:
To balance the given equation in acidic medium, we need to follow the steps of the half-reaction method:
- Balance the atoms other than H and O.
- Balance the O atoms by adding
 molecules.
molecules. - Balance the H atoms by adding H+ ions.
- Balance the charges by adding electrons (e-).
- Ensure that the number of electrons transferred in each half-reaction is the same.
- Add the two half-reactions together and cancel out any common terms.
- Check that both mass and charge are balanced.
Using these steps, the balanced equation for the given reaction in acidic medium is: