Answer: The statement the
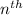 shell holds a minimum number of
shell holds a minimum number of
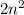 electrons is true.
electrons is true.
Step-by-step explanation:
The alphabet 'n' denotes the principal quantum number whose value can be equal to 1, 2, 3, and so on but can never be zero.
Basically, 'n' is the number of shell of an atom and total number of orbitals present in a shell is
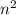 .
.
Each orbital can hold up to a maximum of wo electrons. Hence, the
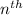 shell can hold a minimum number of
shell can hold a minimum number of
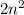 electrons.
electrons.
Thus, we ca conclude that the statement the
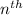 shell holds a minimum number of
shell holds a minimum number of
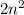 electrons is true.
electrons is true.