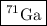Answer:
symbol:
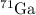 or Ga-71
or Ga-71
mass: 70.94 u
abundance: 39.53%
Step-by-step explanation:
We are given the following information:
- The atomic weight (average atomic mass) of the metal Gallium is 69.72 u (unified atomic mass units).
- Gallium has 2 naturally occurring isotopes.
- One of these is
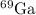 , which has a mass of 68.9257 u and an abundance of 60.47%.
, which has a mass of 68.9257 u and an abundance of 60.47%.
We are solving for the mass and abundance of the other isotope (
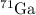 ).
).
First, we can calculate its abundance by subtracting the abundance of the other isotope (
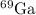 ) from 100%:
) from 100%:
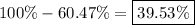
So, the abundance of the other isotope is 39.53%.
Next, we can model the average atomic mass calculation with the equation:
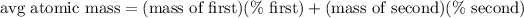
↓ plugging in the known values
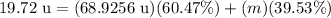
Now, we can solve for m (the mass of the second isotope) by isolating it on one side of the equation:
↓ subtracting
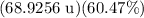 from both sides
from both sides
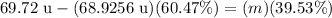
↓ dividing both sides by 39.53%
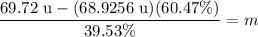
↓ evaluating using a calculator

We can round this value to get the mass number (# protons + # neutrons) of the second isotope:
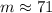
Therefore, the symbol for the second isotope is: