Final answer:
The
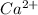 ion will elevate the boiling point of
ion will elevate the boiling point of
 the most because it dissociates to give three ions per formula unit, leading to a higher elevation compared to Na+ and K+ which only produce two ions per formula unit.
the most because it dissociates to give three ions per formula unit, leading to a higher elevation compared to Na+ and K+ which only produce two ions per formula unit.
Step-by-step explanation:
The ion that will contribute most to elevating the boiling point of H₂O among Na+, K+, and
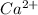 is
is
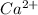 . This is because the boiling point elevation of a solution is proportional to the molal concentration of the dissolved particles (ions in this case), a concept known as the colligative properties of solutions.
. This is because the boiling point elevation of a solution is proportional to the molal concentration of the dissolved particles (ions in this case), a concept known as the colligative properties of solutions.
Since each mole of CaCl₂ dissociates into three ions (one
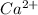 and two Cl−), it will contribute more to the elevation of the boiling point compared to NaCl and KCl, which dissociate into two ions each.
and two Cl−), it will contribute more to the elevation of the boiling point compared to NaCl and KCl, which dissociate into two ions each.