Answer:
Step-by-step explanation:
This is a second order reaction, so we will use the integrated rate law for a second order reaction, then rearrange it to solve for concentration after time t:
![(1)/([A]t)](https://img.qammunity.org/2024/formulas/chemistry/high-school/a029ksc0tnstxxgfu0skrvstbteebrmimf.png) -
-
![(1)/([A]init)](https://img.qammunity.org/2024/formulas/chemistry/high-school/wlq0yfycw4786hs84a3snucirzcwor5dvp.png) = kt
= kt
![(1)/([A]t)](https://img.qammunity.org/2024/formulas/chemistry/high-school/a029ksc0tnstxxgfu0skrvstbteebrmimf.png) = kt +
= kt +
![(1)/([A]init)](https://img.qammunity.org/2024/formulas/chemistry/high-school/wlq0yfycw4786hs84a3snucirzcwor5dvp.png)
[A]t refers to the concentration after time t, and [A]init refers to the initial concentration.
We know k, t, and [A]init, so plug those into the equation:
![(1)/([A]t)](https://img.qammunity.org/2024/formulas/chemistry/high-school/a029ksc0tnstxxgfu0skrvstbteebrmimf.png) = (0.117M⁻¹*min⁻¹)(50.5 min) +
= (0.117M⁻¹*min⁻¹)(50.5 min) +
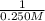
= 5.9085M⁻¹ + 4M⁻¹
= 9.9085M⁻¹
= 1 / 9.9085M⁻¹
[A]t = 0.101M
I hope this helps! :)