Answer: 9.13g NO
Step-by-step explanation:
First, I list what I know, which is the values of STP, the volume, the constant R, and the molar mass of NO; I also include what I'm looking for, which is n, the number of moles of NO.
STP = 273.15K, 1 atm
P = 1 atm
V = 6.82L
R = 0.0821 L*atm/mol*K
T = 273.15K
n = ?
NO mass: 30.01 g/mol
Then, I use the ideal gas law, PV = nRT, to find the number of moles of NO:
(1 atm)(6.82 L) = n(
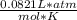 )(273.15K)
)(273.15K)
(1 atm)(6.82 L) = n(
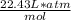 )
)
(1 atm)(6.82 L) / (
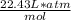 ) = n => divided both sides by 22.4L*atm/mol
) = n => divided both sides by 22.4L*atm/mol
n = 0.304 mol NO
Then, I use the molar mass of NO, 30.01 g/mol, to convert from mol NO to g NO:
g NO = 0.304 mol NO(
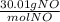 )
)
= 9.13 g NO
I hope this helps! :)