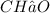Final answer:
To calculate the molecular formula from the empirical formula
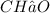 molar mass of 15.0 g/mol, we divide the molar mass by the empirical formula mass and round to the nearest whole number. The molecular formula of the compound is
molar mass of 15.0 g/mol, we divide the molar mass by the empirical formula mass and round to the nearest whole number. The molecular formula of the compound is
Step-by-step explanation:
To calculate the molecular formula from the empirical formula, we need to know the molar mass of the compound. In this case, the molar mass is given as 15.0 g/mol. We can start by finding the empirical formula mass of
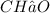 rbon has a molar mass of 12.01 g/mol, hydrogen has a molar mass of 1.01 g/mol, and oxygen has a molar mass of 16.00 g/mol. So the empirical formula mass is 12.01 + 2(1.01) + 16.00 = 30.03 g/mol.
rbon has a molar mass of 12.01 g/mol, hydrogen has a molar mass of 1.01 g/mol, and oxygen has a molar mass of 16.00 g/mol. So the empirical formula mass is 12.01 + 2(1.01) + 16.00 = 30.03 g/mol.
To find the molecular formula, we divide the molar mass of the compound (15.0 g/mol) by the empirical formula mass (30.03 g/mol). The result is 0.4997. Since the molecular formula should be a whole number multiple of the empirical formula, we round 0.4997 to the nearest whole number, which is 1.
Therefore, the molecular formula of the compound is the same as the empirical formula,