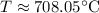The estimated temperature at which the reaction begins to favor the formation of products is approximately 708.05°C.
To estimate the temperature at which the reaction begins to favor the formation of products, we can use the concept of Gibbs free energy (ΔG) and the relationship:
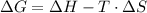
At equilibrium, ΔG=0. Therefore, we can rearrange the equation to solve for the temperature (T):
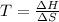
Where:
ΔH is the enthalpy change of the reaction.
ΔS is the entropy change of the reaction.
We need to refer to thermodynamic data to obtain ΔH and ΔS for the reaction:
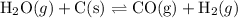
Using the standard enthalpies of formation
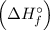 and standard entropies
and standard entropies
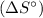 for each species, we can calculate ΔH and ΔS.
for each species, we can calculate ΔH and ΔS.
For standard thermodynamic data, we have:
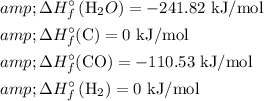
Let's calculate ΔH using these values:
![\begin{aligned}& \Delta H=\sum \Delta H_f^(\circ)(\text { products })-\sum \Delta H_f^(\circ)(\text { reactants }) \\& \Delta H=[-110.53+0]-[(-241.82+0)] \mathrm{kJ} / \mathrm{mol} \\& \Delta H \approx 131.29 \mathrm{~kJ} / \mathrm{mol}\end{aligned}](https://img.qammunity.org/2024/formulas/chemistry/high-school/jmvyisazkakk9wubokbfpl0qjio4j9pjo3.png)
For the entropy change ΔS, we can use standard entropy values:
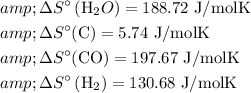
![\begin{aligned}& \Delta S=\sum \Delta S^(\circ)(\text { products })-\sum \Delta S^(\circ)(\text { reactants }) \\& \Delta S=[197.67+130.68]-[188.72+5.74] \mathrm{J} / \mathrm{mol} \mathrm{K} \\& \Delta S \approx 133.89 \mathrm{~J} / \mathrm{mol} \mathrm{K}\end{aligned}](https://img.qammunity.org/2024/formulas/chemistry/high-school/21bq5itmjyks3b37qng2o17vx88ngluadz.png)
Now, substitute these values into the temperature equation:
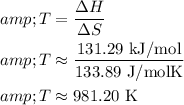
Convert to Celsius: