The estimated value for
 is approximately -2957 kJ/mol.
is approximately -2957 kJ/mol.
The formation of magnesium fluoride
 from its elements involves various steps:
from its elements involves various steps:
1.
 (First ionization energy)
(First ionization energy)
2.
 (Second ionization energy)
(Second ionization energy)
3.
 (Bond dissociation of
(Bond dissociation of
 )
)
4.
 (Electron affinity of fluorine)
(Electron affinity of fluorine)
5.
 (Enthalpy of sublimation for Mg)
(Enthalpy of sublimation for Mg)
6.
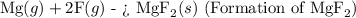
The enthalpy change for the formation of
 can be determined using Hess's Law by considering these steps.
can be determined using Hess's Law by considering these steps.
Given:
Lattice energy = -2913 kJ/mol
First ionization energy of Mg = 735 kJ/mol
Second ionization energy of Mg = 1445 kJ/mol
Electron affinity of F = -328 kJ/mol
Bond energy of F2 = 154 kJ/mol
Enthalpy of sublimation for Mg = 150 kJ/mol
The enthalpy change for the formation of
 can be calculated as follows:
can be calculated as follows:
![\[ \Delta H^\circ_f (\text{MgF}_2) = \text{Lattice energy} + \text{IE}_1(\text{Mg}) + \text{IE}_2(\text{Mg}) + \text{EA}(\text{F}) + \text{Bond energy} - \text{Sublimation energy} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/82f2pfa40jfr6w7h7jhohbppi29b86o3an.png)
Substitute the given values:
![\[ \Delta H^\circ_f (\text{MgF}_2) = -2913 + 735 + 1445 - 328 + 154 - 150 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/k3ah2rxlhrwpcxtqwgdcx3oh34jvr1u45f.png)
Sure, let's calculate the enthalpy change for the formation of magnesium fluoride
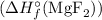 using the given values:
using the given values:
![\[\Delta H^\circ_f (\text{MgF}_2) = -2913 + 735 + 1445 - 328 + 154 - 150\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/q9wq2jiqjswshxn55mjdw2o36xhseytq16.png)
![\[\Delta H^\circ_f (\text{MgF}_2) = -2957 \, \text{kJ/mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/i4oq968d1pl1nqztzcd0kq9h6uvmg3vkn3.png)