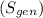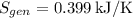The total heat transfer to the water is approximately 784.385 kJ, and the entropy generation during the process is about 0.399 kJ/K. These calculations are based on standard steam table values and assume a final state with an equal mix of saturated liquid and vapor.
To solve this problem, we'll follow these steps:
1. Determine the initial state of the water.
2. Determine the final state of the water.
3. Calculate the total heat transfer.
4. Calculate the entropy generation.
Step 1: Determine the Initial State of the Water
- Initial pressure,
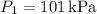
- Initial temperature,
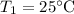
- Mass of water,
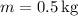
At 25°C and 101 kPa, water is in a compressed liquid state (subcooled liquid). We'll use the properties of water at this state.
Step 2: Determine the Final State of the Water
- The pressure required to float the piston is 1000 kPa, but since the volume becomes five times the initial value, the piston has moved, and we have a two-phase mixture.
- Final pressure,
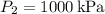
Step 3: Calculate the Total Heat Transfer
1. Calculate the initial internal energy
 :
:
We'll use the specific internal energy of water at 25°C (from steam tables).
2. Calculate the final internal energy
 :
:
At 1000 kPa, find the properties of saturated liquid
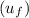 and saturated vapor
and saturated vapor
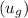 from the steam table. Use these to calculate
from the steam table. Use these to calculate
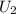 for the two-phase mixture.
for the two-phase mixture.
3. Heat Transfer
 :
:
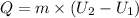
Step 4: Calculate the Entropy Generation
1. Calculate the initial entropy
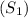 :
:
Use the specific entropy of water at 25°C.
2. Calculate the final entropy
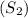 :
:
At 1000 kPa, find the properties of saturated liquid
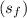 and saturated vapor
and saturated vapor
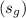 from the steam table. Use these to calculate
from the steam table. Use these to calculate
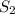 .
.
3. Entropy Generation
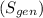 :
:
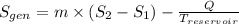
Where
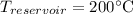 (converted to Kelvin).
(converted to Kelvin).
Let's start by finding the specific internal energy and specific entropy values from the steam tables for the initial and final states. Then, we'll proceed with the calculations.
Here are the results from the calculations:
1. Initial State Properties (at 25°C, Compressed Liquid)
- Specific Internal Energy,
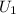 :
:
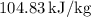
- Specific Entropy,
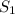 :
:
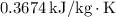
2. Final State Properties (at 1000 kPa, Two-Phase Mixture)
Specific Internal Energy,
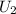 :
:
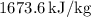
Specific Entropy,
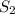 :
:
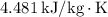
3. Total Heat Transfer

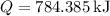
4. Entropy Generation