Answer:
Using PV=nRT
- since
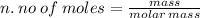
- PV= nRT
P=775mmhg/760mmhg
P=1.01atm
so,n=PV/RT
n= (1.01*0.102)/(0.0821*373)
n= 0.00339mole
n =mass/ molecular mass
molecular mass =0.265/0.00339
molecular mass= 78.17
empirical formular = C. H
C= 92.24. 92.24/12= 7.688
H= 7.76. 7.76/1=7.76
therefore Empirical formula is CH
(CH)n=78.16
(12+1)n=78.14
n=78.14/13
n= 6.01
therefore, molecular formula is
C6H6