Answer: 5.04g C
Step-by-step explanation:
This is a unit conversion problem, specifically a mole-gram conversion, so we will be converting from mol C (moles of carbon) to g C (grams of carbon) using our given number of moles and the molar mass of carbon.
0.420mol C (
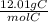 ) = 5.04g C
) = 5.04g C
We're multiplying mol by
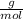 , so the mol cancels out, leaving our desired unit, grams.
, so the mol cancels out, leaving our desired unit, grams.
I hope this helps! :)