Answer: 107.865 amu
Step-by-step explanation:
To find the atomic mass of silver, we first multiply each isotopic mass by their % abundance, add these values together, then divide by 100.
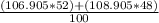 = 107.865 amu
= 107.865 amu
So, the atomic mass of silver is 107.865 amu, which makes sense because it's between the two isotopic masses of 106.905 amu and 108.905 amu.
I hope this helps! :)