Answer: 8502.2 mol IO₂
Step-by-step explanation:
This is a unit conversion problem, and we will be converting molecules of IO₂ to moles IO₂ using Avogadro's constant (6.022 x 10²³ molecs/1 mol).
I wrote it two different ways in case the fraction can be a bit difficult to read. :)
5.12 x 10²⁷ molecs IO₂ (
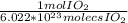 ) = 8502.2 mol IO₂
) = 8502.2 mol IO₂
5.12 x 10²⁷ molecs IO₂ (1 mol IO₂/6.022 x 10²³ molecs IO₂) = 8502.2 mol IO₂
I hope this helps! :)