The temperature of the cloth after evaporative cooling is approximately 24.72 degrees Celsius.
To determine the temperature of the cloth wrapped around the head of a desert dweller, we need to consider the process of evaporative cooling. When the cloth is soaked with water, the water will evaporate from the cloth's surface, absorbing heat energy from the surroundings, including the desert dweller's head. This process cools down the cloth.
Here are the steps to calculate the temperature of the cloth:
1. Given Information:
- Atmospheric pressure (P) = 1 atm
- Initial temperature (Tinitial) = 45°C
- Relative humidity (RH) = 20%
2. Convert Temperature to Kelvin:
- To work with the temperature in the Kelvin scale, convert the initial temperature from Celsius to Kelvin using the following formula:
T(K) = T(°C) + 273.15
- T(K) = 45°C + 273.15 = 318.15 K
3. Determine Saturation Vapor Pressure:
- Find the saturation vapor pressure (Psat) at the given temperature (Tinitial). You can use a vapor pressure table or an equation. One common equation is the Antoine equation, which varies depending on the substance (water in this case). For water at this temperature, P_sat is approximately 0.07176 atm.
4. Calculate Actual Vapor Pressure:
- Since the relative humidity (RH) is 20%, the actual vapor pressure (Pactual) of water in the air is 20% of Psat.
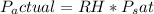
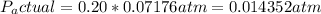
5. Calculate Temperature After Evaporative Cooling:
- Use the Clausius-Clapeyron equation to calculate the final temperature (Tfinal) after evaporative cooling:
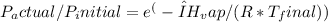
Where:
- Pactual is the actual vapor pressure.
- Pinitial is the initial pressure (1 atm).
- ΔHvap is the enthalpy of vaporization of water.
- R is the ideal gas constant (8.314 J/(mol·K)).
- Tfinal is the final temperature in Kelvin.
- Rearrange the equation to solve for Tfinal:
Tfinal = -ΔH_vap / (R * ln(P_actual / P_initial))
- ΔH_vap for water is approximately 40.7 kJ/mol.
- Plug in the values and calculate:
Tfinal = -(40.7 kJ/mol) / (8.314 J/(mol·K) * ln(0.014352 atm / 1 atm))
Tfinal ≈ 297.87 K
6. Convert Final Temperature to Celsius:
- Convert the final temperature from Kelvin to Celsius using the formula:
Tfinal(°C) = Tfinal(K) - 273.15
- Tfinal(°C) ≈ 297.87 K - 273.15 ≈ 24.72°C