Answer:
0.043 grams
Step-by-step explanation:
We can find the mass of carbon dioxide as follows:
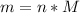
Where:
n: is the number of moles
M: is the molar mass = 44.01 g/mol
First, we need to calculate the number of moles. We can use the Ideal gas equation:
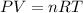
Where:
P: is the pressure = 0.945 atm
V: is the volume = 25.0 mL
R: is the gas constant = 0.082 L*atm/(K*mol)
T: is the tempearture = 22.5 °C
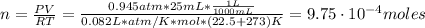
Hence, the mass is:

Therefore, 0.043 grams were collected.
I hope it helps you!