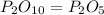The empirical formula for the given molecular formula
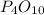 PO is
PO is
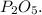
The empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound.
We start by dividing the subscripts of each element by the greatest common factor (GCF).: The GCF for phosphorus (P) is 2 and for oxygen (O) is 1.
Divide the subscripts of phosphorus by 2: P4 / 2 = P2
Divide the subscripts of oxygen by 1: O10 / 1 = O10 (already at its simplest form)
Combine the simplified subscripts: