Answer:
m = 1.2 g
Step-by-step explanation:
Density
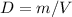 where D is density, m is mass and V is volume.
where D is density, m is mass and V is volume.
We're given:
First, convert mL to L:
1500 mL = 1.5 L
Then, multiply the volume by density to find mass:
m = 0.78 × 1.5
m = 1.17 g
Since all the given values had 2 significant figures, round our answer to 2 significant figures:
m = 1.2 g