Answer:
1:25
Step-by-step explanation:
Rate of Diffusion & Molar Mass
We're given:
- gas P diffuses 5 times faster than gas Q
Let
 be the rate of diffusion of gas P, and let
be the rate of diffusion of gas P, and let
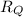 be the rate of diffusion of gas Q.
be the rate of diffusion of gas Q.
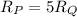
Rate of diffusion is inversely proportional to molar mass:
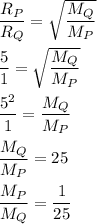
Thus, the ratio of molecular mass of P to Q is 1:25.