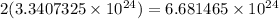Answer:
# O atoms =
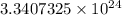
# H atoms =
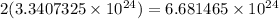
Step-by-step explanation:
Stoichiometry
We're given:
- 100 g of H2O
- # H atoms?
- # O atoms?
1) Find the moles of H2O.
 where n is moles, m is mass and M is molar mass
where n is moles, m is mass and M is molar mass
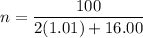
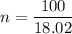
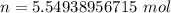
2) Find the number of molecules of H2O.
n × Avogadro's number = # atoms
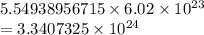
∴ There are
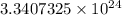 molecules of H2O.
molecules of H2O.
3) Find the number of atoms of H and O.
There are 2 H atoms and 1 O atom in every molecule of H2O.
Therefore...
# O atoms =
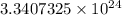
# H atoms =