Cyclohexene shows a positive test result as it undergoes oxidation, leading to the fading of the purple color of
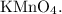 Cyclohexane, on the other hand, does not undergo a noticeable reaction with
Cyclohexane, on the other hand, does not undergo a noticeable reaction with
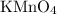 and shows a negative test result. The test results for the unknown compound would need experimental evaluation.
and shows a negative test result. The test results for the unknown compound would need experimental evaluation.
The potassium permanganate test is used to observe the oxidation reactions of different compounds. When reacting with certain organic compounds, potassium permanganate
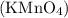 undergoes reduction, resulting in a color change or other observable reactions.
undergoes reduction, resulting in a color change or other observable reactions.
Here are the typical observations and test results for the compounds mentioned:
1. Cyclohexene:
- Observation: Decolorization or disappearance of the purple color of
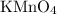 solution.
solution.
- Test Result: Positive. The purple color of
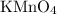 solution fades or disappears due to the oxidation of cyclohexene.
solution fades or disappears due to the oxidation of cyclohexene.
2. Cyclohexane:
- Observation: No significant change or reaction.
- Test Result: Negative. There is no observable reaction with
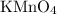 solution, as cyclohexane is relatively inert to oxidation.
solution, as cyclohexane is relatively inert to oxidation.
3. Unknown Compound:
- The observations and test results for the unknown compound would need to be determined through experimentation or comparison with known compounds. The reaction would depend on the specific chemical nature of the unknown compound and how it interacts with potassium permanganate.
Question: