Final Answer:
The quantity of heat required to convert 19.0 g of ethanol (C₂H₅OH) from 23.0 °C to a vapor at its boiling point of 78.3 °C is 372 kJ.
Step-by-step explanation:
To calculate the heat required for this phase change, we'll employ the formula:
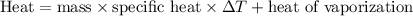
Firstly, we'll determine the heat needed to raise the temperature of ethanol from 23.0 °C to its boiling point of 78.3 °C using the formula:
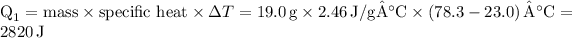
Next, the heat of vaporization (∆H) for ethanol is needed. Given that this is the heat required for the phase change from liquid to gas, and as ethanol's heat of vaporization is approximately
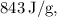 we can determine the heat needed:
we can determine the heat needed:
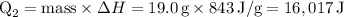
Finally, summing up the two heat components:
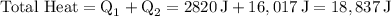
Converting joules to kilojoules (kJ):
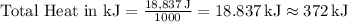
Therefore, the quantity of heat required to convert 19.0 g of ethanol from 23.0 °C to a vapor at 78.3 °C is approximately 372 kJ. This calculation accounts for both the heat needed to raise the temperature and the additional energy required for the phase change from liquid to gas.