Therefore, after the addition of
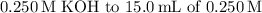 hypobromous acid, the pH of the resulting solution is approximately
hypobromous acid, the pH of the resulting solution is approximately
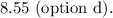
This problem involves the titration of a weak acid (hypobromous acid, HBrO) with a strong base (KOH). The initial concentration of hypobromous acid and the volume of the base added allow us to determine the pH of the resulting solution after the addition of the base.
Firstly, let's establish the balanced chemical equation for the reaction between hypobromous acid and potassium hydroxide:
![\[ \text{HBrO} + \text{KOH} \rightarrow \text{KBrO} + \text{H}_2\text{O} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/vzsegjryhafqp1dfryuxwth9svyofpinwb.png)
In this titration, the initial concentration of hypobromous acid is
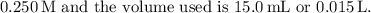 As the reaction proceeds, the moles of base added will neutralize the moles of acid.
As the reaction proceeds, the moles of base added will neutralize the moles of acid.
Since both the acid and base have the same concentration and volume, they will neutralize each other completely. This results in a solution of the potassium salt of hypobromous acid, which dissociates in water.
To find the pH after the reaction, we need to determine the moles of acid initially present and the moles of base added:
![\[ \text{Moles of acid} = \text{M} * \text{Volume} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/7tztob0hw511sg4tl5777ydxn1ldyellit.png)
![\[ \text{Moles of acid} = 0.250 \, \text{M} * 0.015 \, \text{L} = 0.00375 \, \text{mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/82ipl0fvwjb308go1zqitfph467r6vyba0.png)
This amount of acid will be neutralized by the same number of moles of the base because they're in a 1:1 ratio.
Now, the total volume of the solution after titration is
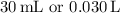 (initial volume of acid + volume of base added).
(initial volume of acid + volume of base added).
To calculate the concentration of the resulting solution:
![\[ \text{Total moles} = \text{Moles of acid} + \text{Moles of base} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/g4o8j52tofaodqrmp5d7pjvuzjzc8wnf7s.png)
![\[ \text{Total moles} = 0.00375 \, \text{mol} + 0.00375 \, \text{mol} = 0.0075 \, \text{mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/el2qshgn2uduzlrabtrqyip37q03mnfplv.png)
The total volume is
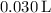 , so the concentration of the resulting solution is:
, so the concentration of the resulting solution is:
![\[ \text{Molarity} = \frac{\text{Total moles}}{\text{Total volume}} = \frac{0.0075 \, \text{mol}}{0.030 \, \text{L}} = 0.25 \, \text{M}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/8r776tpivkzz5fixe2extfygcj11qoxc2w.png)
This concentration represents the concentration of the hypobromite ion,
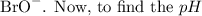 of this solution, we can use the dissociation of the hypobromite ion:
of this solution, we can use the dissociation of the hypobromite ion:
![\[ \text{BrO}^- + \text{H}_2\text{O} \rightleftharpoons \text{HBrO} + \text{OH}^- \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/sh52cy9ioqtudft60phz6owdj49q0nzazd.png)
Given the
 of hypobromous acid
of hypobromous acid
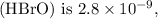 we can set up an ICE table to determine the concentration of
we can set up an ICE table to determine the concentration of
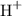 ions and subsequently find the pH.
ions and subsequently find the pH.
The
 expression is:
expression is:
![\[ K_a = \frac{\text{[HBrO][OH}^-]}{\text{[BrO}^-]} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/852v0jkmbpveustplpd9scwajucopwh2tc.png)
At the equivalence point, the concentration of
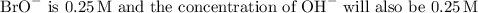 due to the complete neutralization of the acid by the base.
due to the complete neutralization of the acid by the base.
![\[ K_a = \frac{\text{[H][OH}^-]}{\text{[BrO}^-]} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/f7mj1b0yockkdbizo73swlzttk3s35wgse.png)
As the concentration of
 is known, we can calculate the concentration of
is known, we can calculate the concentration of
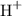 ions:
ions:
![\[ K_a = \frac{\text{[H][0.25]}}{0.25} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/y1syvnfrb5ibnl2d51qbyl71ove1hub7bd.png)
![\[ \text{[H]} = K_a \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/a4lhw8j1ha158j7nfwv42reknumhxd7kuv.png)
Now, take the negative logarithm of
![\(\text{[H]}\) to find the \(pH\):](https://img.qammunity.org/2024/formulas/chemistry/high-school/27slhlp6eb9pnmb2i5getsvj2xmwhwbgpm.png)
![\[ pH = -\log(\text{[H]}) = -\log(K_a) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/wt287jpazwbmac2c5kf1vx3rxbcz8bxqhq.png)
![\[ pH = -\log(2.8 * 10^(-9)) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/t1yx8p8o0pyklk9ujjkgk90l8sp16m26by.png)
Calculate pH:
![\[ pH = -\log(2.8 * 10^(-9)) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/t1yx8p8o0pyklk9ujjkgk90l8sp16m26by.png)
![\[ pH \approx 8.55 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/ygfbbwsqn09t0vek4q75u29hi7jitchaiz.png)