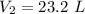Answer:
23.2 L
Step-by-step explanation:
Boyle's Law
Boyle's Law states that pressure and volume are inversely proportional when it comes to ideal gases.

- P1, V1 = initial pressure and volume
- P2, V2 = final pressure and volume
We're given:
- V1 = 22.5 L
- P1 = 748 mmHg
- V2 = ?
- P2 = 725 mmHg

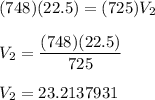
Round to 3 significant figures: