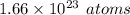Answer:
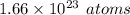
Step-by-step explanation:
Avogadro's Number
We're given:
First, determine moles (n):
 where m is mass and M is molar mass
where m is mass and M is molar mass
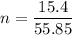 (molar mass taken from a periodic table)
(molar mass taken from a periodic table)
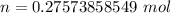
Now, multiply the moles by Avogadro's number to find atoms:
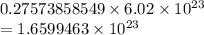
Round to 3 significant figures: