The theoretical yield of barium sulfate is approximately 4.41 grams, and the percent yield is approximately 88.66%.
To determine the theoretical yield of barium sulfate, we need to find the stoichiometric ratio between barium sulfate and potassium sulfate in the balanced chemical equation. The balanced equation is:
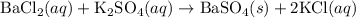
The molar ratio between barium sulfate
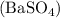 and potassium sulfate
and potassium sulfate
 is 1:1. Therefore, the moles of barium sulfate formed should be equal to the moles of potassium sulfate reacted.
is 1:1. Therefore, the moles of barium sulfate formed should be equal to the moles of potassium sulfate reacted.
First, calculate the moles of potassium sulfate
 using its molar mass:
using its molar mass:

Now, calculate the moles of
 :
:
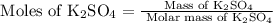
Next, since the molar ratio is 1:1 between
 and
and
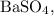 the moles of
the moles of
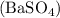 formed are the same.
formed are the same.
Now, calculate the theoretical yield of
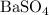 using its molar mass:
using its molar mass:
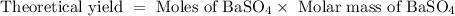
Finally, calculate the percent yield using the formula:
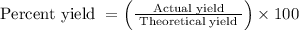
To calculate the theoretical yield and percent yield, we need to follow the steps outlined above.
Calculate moles of potassium sulfate
 :
:
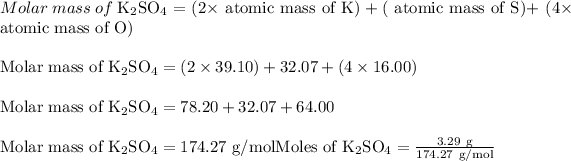
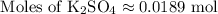
Calculate theoretical yield of barium sulfate
 :
:
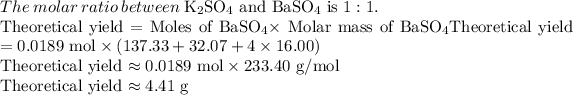
Calculate percent yield:

Question:
For the following reaction, 3.29 grams of potassium sulfate are mixed with excess barium chloride. The reaction yields 3.91 grams of barium sulfate.
barium chloride (aq) + potassium sulfate (aq) -->barium sulfate (s) + potassium chloride (aq)
What is the theoretical yield of barium sulfate ? __ grams
What is the percent yield of barium sulfate ?