The predicted vapor pressure of the solution of 266 mL glycerol and 399 mL water at the normal boiling point of water is approximately
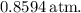
To predict the vapor pressure of the solution, we can use Raoult's law, which states that the vapor pressure of a solution is directly proportional to the mole fraction of each component in the solution.
Given:
- Volume of glycerol = 266 mL
- Volume of water = 399 mL
- Density of glycerol = 1.25 g/mL
First, let's calculate the moles of glycerol and water in the solution:
For glycerol:
![\[ \text{Volume} * \text{Density} = \text{Mass} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/o0tje5yk31ihh5raq21v2z8anlqtfff9ct.png)
![\[ 266 \, \text{mL} * 1.25 \, \text{g/mL} = 332.5 \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/6yoahgts5wjkfv517p9lu7yqrg55lqvc3x.png)
The molar mass of glycerol
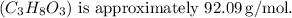
![\[ \text{Moles of glycerol} = \frac{\text{Mass}}{\text{Molar mass}} = \frac{332.5 \, \text{g}}{92.09 \, \text{g/mol}} \approx 3.61 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/5kbomv9dj5sn9vo945am2ohs6n6gf1ky9d.png)
For water:
![\[ \text{Volume} * \text{Density} = \text{Mass} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/o0tje5yk31ihh5raq21v2z8anlqtfff9ct.png)
![\[ 399 \, \text{mL} * 1 \, \text{g/mL} = 399 \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/gb9otiw4cusmetuknbbg04ki785oo7ncg1.png)
The molar mass of water
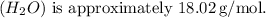
![\[ \text{Moles of water} = \frac{\text{Mass}}{\text{Molar mass}} = \frac{399 \, \text{g}}{18.02 \, \text{g/mol}} \approx 22.17 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/3xvetscd6ego10aeziblw00hw6o21r4sck.png)
Now, let's find the mole fraction of each component:
Mole fraction of glycerol:
![\[ \text{Mole fraction} = \frac{\text{Moles of glycerol}}{\text{Total moles in solution}} = \frac{3.61 \, \text{mol}}{3.61 \, \text{mol} + 22.17 \, \text{mol}} \approx 0.1406 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/7e45woygflo5ep3mj04j7hmzg2lhrqchcu.png)
Mole fraction of water:
![\[ \text{Mole fraction} = \frac{\text{Moles of water}}{\text{Total moles in solution}} = \frac{22.17 \, \text{mol}}{3.61 \, \text{mol} + 22.17 \, \text{mol}} \approx 0.8594 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/22ag3a50po92nlv2c4tew9ogxnad6gtz8f.png)
Now, we'll use Raoult's law:
![\[ P_{\text{total}} = P_{\text{glycerol}} * \text{mole fraction of glycerol} + P_{\text{water}} * \text{mole fraction of water} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/xzulcwyt3yl5kvdln1ickxtoqiwl9zubw3.png)
At the normal boiling point of water, the vapor pressure of pure water is
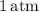 (since it's boiling at its normal boiling point).
(since it's boiling at its normal boiling point).
So, the vapor pressure of the solution would be:
![\[ P_{\text{total}} = 1 \, \text{atm} * 0.8594 + P_{\text{glycerol}} * 0.1406 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/i5dip4fk7ee5a51ilk5bty0wol6hmndubg.png)
We need to find the vapor pressure of glycerol to calculate
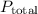 . The vapor pressure of glycerol can be considered negligible compared to water at its boiling point. Therefore:
. The vapor pressure of glycerol can be considered negligible compared to water at its boiling point. Therefore:
![\[ P_{\text{total}} \approx 1 \, \text{atm} * 0.8594 = 0.8594 \, \text{atm} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/b9391r6o4f04i2ltv7795r8o8ucl8z3hvo.png)