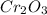Final answer:
The empirical formula of Chromium Oxide is found by converting the masses of chromium and oxygen to moles, finding the simplest mole ratio, and then adjusting it to get whole numbers. The final empirical formula is Cr₂O₃.
Step-by-step explanation:
The question is asking to determine the empirical formula of Chromium Oxide, which involves finding the simplest whole number ratio of the elements in the compound. To do this, we first convert the given masses of chromium and oxygen into moles by using their molar masses. According to the question, Chromium (Cr) has a molar mass of 52.00 g/mol and Oxygen (O) has a molar mass of 16.00 g/mol.
For Chromium:
10.4 g Cr × (1 mol Cr / 52.00 g Cr) = 0.2 mol Cr
For Oxygen:
Mass of O = Total mass of Chromium Oxide - Mass of Cr = 15.2 g - 10.4 g = 4.8 g O4.8 g O × (1 mol O / 16.00 g O) = 0.3 mol O
Now, we calculate the simplest whole number ratio of moles of chromium to moles of oxygen by dividing each by the smallest mole value, which in this case is for chromium (0.2 mol).
Ratio of Cr:O:
0.2 mol Cr / 0.2 mol = 1
0.3 mol O / 0.2 mol = 1.5
Since we usually do not use fractional subscripts in empirical formulas, we multiply each by 2 to get whole numbers: 1 × 2 = 2 for Cr and 1.5 × 2 = 3 for O, resulting in an empirical formula of
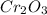
Therefore, the empirical formula of Chromium Oxide is