The molecule
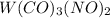 is called tricarbonyl nitrosyl dinitrosyltungsten.
is called tricarbonyl nitrosyl dinitrosyltungsten.
The molecule
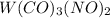 is a coordination complex containing tungsten (W), carbon monoxide (CO), and nitric oxide (NO) ligands.
is a coordination complex containing tungsten (W), carbon monoxide (CO), and nitric oxide (NO) ligands.
- W: Represents the chemical symbol for tungsten, a transition metal.
-
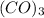 : Indicates three carbon monoxide molecules acting as ligands. Ligands are molecules or ions that bond to a central metal atom in a complex.
: Indicates three carbon monoxide molecules acting as ligands. Ligands are molecules or ions that bond to a central metal atom in a complex.
-
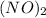 : Denotes two nitric oxide molecules also acting as ligands, bonding to the central tungsten atom.
: Denotes two nitric oxide molecules also acting as ligands, bonding to the central tungsten atom.
This molecule is an example of a transition metal complex where the central tungsten atom is surrounded by different ligands. The combination of these ligands and the central metal atom forms a coordination complex with specific properties and characteristics.