Final Answer:
The temperature of the krypton gas sample at the new volume of 10.6 L and pressure of 0.846 atm is 70.5 °C.
Step-by-step explanation:
To find the new temperature, we can use the combined gas law, which relates the initial and final states of a gas sample under changing conditions. The combined gas law is given by the equation:
![\[ (P_1 \cdot V_1)/(T_1) = (P_2 \cdot V_2)/(T_2) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/8vrc7t497eupsk88qfhvkwnin0w57cdmcc.png)
where
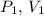 , and
, and
 are the initial pressure, volume, and temperature, respectively, and \( P_2 \), \( V_2 \), and \( T_2 \) are the final pressure, volume, and temperature.
are the initial pressure, volume, and temperature, respectively, and \( P_2 \), \( V_2 \), and \( T_2 \) are the final pressure, volume, and temperature.
In this case, the initial conditions are
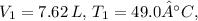 and
and
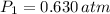 . The final conditions are
. The final conditions are
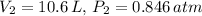 , and we need to find
, and we need to find
 .
.
Rearranging the combined gas law to solve for
 , we get:
, we get:
![\[ T_2 = (P_2 \cdot V_2 \cdot T_1)/(P_1 \cdot V_1) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/bvcgmn453800nwicnecm5ca2ppy1maxuyt.png)
Plugging in the values, we find:
![\[ T_2 = ((0.846 \, atm) \cdot (10.6 \, L) \cdot (49.0 °C + 273.15))/((0.630 \, atm) \cdot (7.62 \, L)) \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/i5soxbitrh7popghbsvbcbexxzah2xwocc.png)
After solving, we get
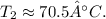
Therefore, the temperature of the krypton gas at the new volume and pressure is approximately 70.5 °C.