Answer:
Molar mass = 63.54 approximately
Step-by-step explanation:
In order to find the molar mass of a sample of naturally occurring copper, we can use the weighted average of the molar masses of its isotopes, taking into account their relative abundances. The formula for calculating the weighted average molar mass is:
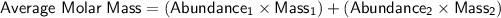
Given the information provided:
Isotope 1:
- Mass: 62.9296 g/mol
- Abundance: 69.200% = 0.692
Isotope 2:
- Mass: 64.9278 g/mol
- Abundance: 30.800% = 0.308
Let's plug in the values and calculate:
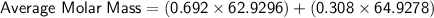
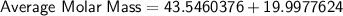
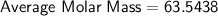
So, the molar mass of a sample of naturally occurring copper is approximately. 63.54.