Answer:
Oxidation state of chromium in
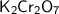 is +6.
is +6.
Step-by-step explanation:
In
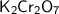 , there are two chromium atoms, two potassium atoms, and seven oxygen atoms.
, there are two chromium atoms, two potassium atoms, and seven oxygen atoms.
The oxidation state of potassium is +1, the oxidation state of oxygen is -2, and the overall charge of the compound is 0.
In this case:
Given:
Oxidation state of potassium (K) = +1
Oxidation state of oxygen (O) = -2
Overall charge of the compound = 0 (since it's neutral)
We can use the following equation based on the oxidation states and the charge:

Plugging in the values for potassium and oxygen:
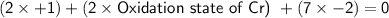
Solving for the oxidation state of chromium:
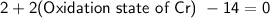



Therefore, in
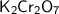 , the oxidation state of chromium is +6.
, the oxidation state of chromium is +6.