Answer:
Molar concentration of NaCl is 2.272 M
Step-by-step explanation:
Molar concentration (also known as molarity) is calculated by dividing the moles of solute by the volume of the solution in liters.
First, let's calculate the moles of NaCl:
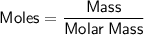
The molar mass of NaCl (sodium chloride) is approximately 58.44 g/mol.
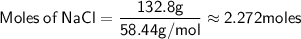
Now, let's convert the volume of the solution to liters:

Now we can calculate the molar concentration (molarity):
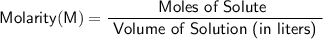
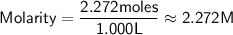
So, the molar concentration of NaCl in the solution is approximately 2.272 M.