Answer:
0.2 moles of butane
Step-by-step explanation:
In order to answer your question, we need to use the balanced chemical equation for the combustion of butane.
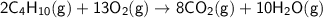
According to the equation, 10 moles of water are produced from 2 moles of butane.
Therefore, we can use a ratio to find the moles of butane that produce 18 g of water:
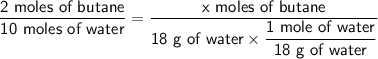
Solving for x, we get:
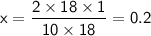
Therefore, 0.2 moles of butane on complete combustion gives 18 g of water.