The percentage (by mass) of
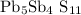 in the sample is approximately 62.32%.
in the sample is approximately 62.32%.
To find the percentage (by mass) of
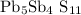 in the sample, we can start by determining the molar mass of
in the sample, we can start by determining the molar mass of
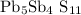 .
.
The molar mass of
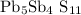 can be calculated by adding the atomic masses of each element in the compound:
can be calculated by adding the atomic masses of each element in the compound:
- Atomic mass of Pb (lead) = 207.2 g/mol
- Atomic mass of Sb (antimony) = 121.76 g/mol
- Atomic mass of S (sulfur) = 32.06 g/mol
Molar mass of
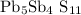 :
:
![\[5 * \text{Atomic mass of Pb} + 4 * \text{Atomic mass of Sb} + 11 * \text{Atomic mass of S}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/65na41s69zrrty9id9ft1tigc92d23sy5x.png)
![\[= 5 * 207.2 \, \text{g/mol} + 4 * 121.76 \, \text{g/mol} + 11 * 32.06 \, \text{g/mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/sxide58byz3j2mclmq93lm5062dkkwquyy.png)
![\[= 1036 + 487.04 + 352.66\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/q21kqp54ex3ku9d2ixy5xhn4fqpvvvegwh.png)
![\[= 1875.7 \, \text{g/mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/dzqf8kh4nup74twaesdyfpeqefuja2cht4.png)
Next, we'll find the number of moles of
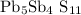 present in the 8.49 g sample:
present in the 8.49 g sample:
Number of moles =
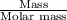
Number of moles
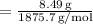
Number of moles ≈
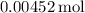
Since the lead in
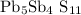 was recovered as
was recovered as
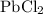 , we need to find the moles of lead in
, we need to find the moles of lead in
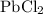 .
.
The molar mass of
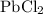 :
:
![\[ \text{Atomic mass of Pb} + 2 * \text{Atomic mass of Cl} = 207.2 \, \text{g/mol} + 2 * 35.45 \, \text{g/mol} = 278.1 \, \text{g/mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/crbm879s499j4hlbegg7luvmylt7c1j5am.png)
Number of moles of
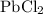 :
:
![\[ \text{Number of moles} = \frac{\text{Mass}}{\text{Molar mass}} = \frac{3.92 \, \text{g}}{278.1 \, \text{g/mol}} = 0.0141 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/g53r0p8xc5yuv356sntic0ulu1cfeu0rgg.png)
Since 1 mol of
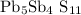 contains 5 moles of lead (Pb), we can calculate the moles of
contains 5 moles of lead (Pb), we can calculate the moles of
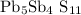 corresponding to the moles of lead obtained:
corresponding to the moles of lead obtained:
![\[ \text{Moles of } \mathrm{Pb}_5 \mathrm{Sb}_4 \mathrm{~S}_(11) = \frac{\text{Number of moles of PbCl}_2}{5} = \frac{0.0141 \, \text{mol}}{5} = 0.00282 \, \text{mol}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/ie0nqxynoe3vh5o9k95yhvp0grud1xkzvu.png)
Finally, let's calculate the mass of
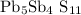 in the sample:
in the sample:
![\[ \text{Mass of } \mathrm{Pb}_5 \mathrm{Sb}_4 \mathrm{~S}_(11) = \text{Number of moles} * \text{Molar mass}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/okd3grushiy9n36nmam3g9p94rqqi4cfjx.png)
![\[ \text{Mass of } \mathrm{Pb}_5 \mathrm{Sb}_4 \mathrm{~S}_(11) = 0.00282 \, \text{mol} * 1875.7 \, \text{g/mol} = 5.29 \, \text{g}\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/4tmrb4o8o7jo3eyn1abdbf1r4u9yvame6l.png)
Now, the percentage (by mass) of
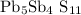 in the sample is:
in the sample is:
![\[ \text{Percentage} = \frac{\text{Mass of } \mathrm{Pb}_5 \mathrm{Sb}_4 \mathrm{~S}_(11)}{\text{Total mass of sample}} * 100\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/91hxhvu2z0zacb8tytpr10w0ula5vugnej.png)
![\[ \text{Percentage} = \frac{5.29 \, \text{g}}{8.49 \, \text{g}} * 100 = 62.32\%\]](https://img.qammunity.org/2024/formulas/chemistry/high-school/hrbepkvmjl0iylv0irednhz5v5zalfo6ql.png)
Therefore, The answer is approximately 62.32%.