The volume of the balloon will remain at 1.50 L even after 0.35 moles of gas are released.
According to Boyle's law:
![\[ P_1V_1 = P_2V_2 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/qxidq6w7llp87rqhn75ywo2lfnlwbe75ro.png)
where
 and
and
 are the initial pressure and volume, and
are the initial pressure and volume, and
 and
and
 are the final pressure and volume.
are the final pressure and volume.
Since the pressure and temperature are held constant, we can simplify the equation to:
At constant pressure:
 , hence, the pressure will cancel out.
, hence, the pressure will cancel out.
Thus, the equation remains:
![\[ V_1 = V_2 \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/egzu1s2dcraaaa1alwbc9qws6t8cf7tzs0.png)
This means that the initial volume
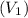 is equal to the final volume
is equal to the final volume
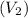 .
.
Therefore, the volume of the balloon will remain at 1.50 L even after 0.35 moles of gas are released, as long as the pressure and temperature remain constant.