In these calculations, we deal with addition, division, multiplication, and conversion of units to calculate the sums, quotients, and number of atoms.
When 88.583 and 74.18 are added:
88.583 + 74.18 = 162.763
When 57.337 is divided by 46.157:
57.337 : 46.157 = 1.24
When 89.55 and 86.910 are multiplied:
89.55 x 86.910 = 7779.2605
When 89.55 and 86.910 are added:
89.55 + 86.910 = 176.460
The radius of a strontium atom is 215 pm. To span a distance of 4.34 mm, we need to convert millimeters to picometers and divide 4.34 mm by the radius of a strontium atom: (4.34 mm * 10000000 pm/mm) / 215 pm = 20186 atoms
The mass of a single chromium atom is
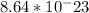 grams. To calculate the number of chromium atoms in 319 milligrams, we need to convert milligrams to grams and divide the mass by the mass of a single chromium atom: (
grams. To calculate the number of chromium atoms in 319 milligrams, we need to convert milligrams to grams and divide the mass by the mass of a single chromium atom: (
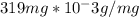 ) / (
) / (
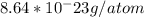 ) =
) =
 atoms
atoms