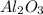Answer:
1.
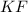
2.
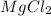
3.
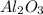
Step-by-step explanation:
To write the correct formula for each compound formed from combining the given pairs of ions, we need to ensure that the compound is electrically neutral. This means the positive and negative charges should cancel each other out.
1. K⁺ and F⁻
- Potassium ion (K⁺) has a charge of +1.
- Fluoride ion (F⁻) has a charge of -1.
- They can combine in a 1:1 ratio to form a neutral compound.
Formula:
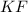
2. Mg²⁺ and Cl⁻
- Magnesium ion (Mg²⁺) has a charge of +2.
- Chloride ion (Cl⁻) has a charge of -1.
- To balance the charges, we need two chloride ions for every magnesium ion.
Formula:
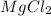
3. Al³⁺ and O²⁻
- Aluminum ion (Al³⁺) has a charge of +3.
- Oxide ion (O²⁻) has a charge of -2.
- To balance the charges, we need two aluminum ions for every three oxide ions.
Formula:
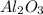
So, the compounds formed are:
1.
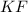
2.
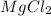
3.