Answer: 3.02 kg of the liquid occupies a volume of
 .
.
Step-by-step explanation:
To find the volume of the liquid, we'll use the formula:
![\[ \text{Volume} = \frac{\text{Mass}}{\text{Density}} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/kvvs66flyzsg5lo4o2k73y1rhmwk413b4o.png)
Given:
Density
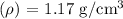
Mass (m) = 3.02 kg = 3020 g (since 1 kg = 1000 g)
Plugging in the values:
![\[ \text{Volume} = \frac{3020 \text{ g}}{1.17 \text{ g/cm}^3} \]](https://img.qammunity.org/2024/formulas/chemistry/college/lm4r7byn0zitfw2jzyzl3jasf7cn6p6mlb.png)
The volume of the liquid is
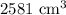 , which is equivalent to
, which is equivalent to
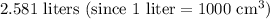 .
.