We know that,

is a reaction which is in equilibrium i.e. the reaction can be proceeded in both directions.
In order to write down the forward reaction, we will simply write ,
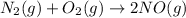
- This reaction being in forward direction,will release heat and therefore, would be termed as an exothermic reaction.
In order to write down the reverse reaction,we will alter the sides of the reaction and it will give the idea that 2 molecules of nitrogen oxide decomposes to give one molecule of dinitrogen and one molecule of oxygen.
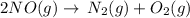
- This reaction being reversed , would absorb heat and hence, would be termed as an endothermic reaction.