Final answer:
The concentration of free Co3+ in the given solution is 4.804e-7 M.
Step-by-step explanation:
To determine the concentration of free Co3+ in the solution, we need to consider the equilibrium of the reaction between Co3+ and NH3. The given reaction is:
![Co3+ + 6 NH3 < -- > [Co(NH3)6]3+](https://img.qammunity.org/2024/formulas/chemistry/high-school/y0kxzekfqm6g9065kc7ylhztkbr6pnghrf.png)
The equilibrium constant (Kf) for this reaction is given as 5.000e+33. We are given the concentrations of Co(NO3)3 and NH3, which are 1.3136e-4 M and 1.2463 M, respectively. We can use the equilibrium expression to determine the concentration of free Co3+.
Since the stoichiometry of the reaction is 1:6 between Co3+ and NH3, the initial concentration of Co3+ is the same as the concentration of
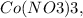 36e-4 M.
36e-4 M.
Let x be the concentration of free Co3+ at equilibrium, then the concentration of
![[Co(NH3)6]3](https://img.qammunity.org/2024/formulas/chemistry/high-school/904q2nitvyh50mayac4w5cnmdlpg85ot7s.png) brium would be (6x)^1/6, and the concentration of NH3 at equilibrium would be 1.2463 - 6x.
brium would be (6x)^1/6, and the concentration of NH3 at equilibrium would be 1.2463 - 6x.
Using the equilibrium expression:
![Kf = ([Co(NH3)6]3+)/(Co3+ * NH3^6)](https://img.qammunity.org/2024/formulas/chemistry/high-school/ourbvqd0hrjf2azr0r7u0kqery4gv24ayt.png)
Substituting the known values:
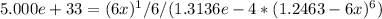
Simplifying the equation, solving for x and plugging in the given values gives:
x = 4.804e-7 M
Therefore, the concentration of free Co3+ in the given solution is 4.804e-7 M.