The correct answer is:
![\[ \text{C. 119.23 g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/8q0y55jsn5o86glcu91p44mmi8mnlaao7s.png) .
.
To find the mass of Ni₃(PO₄)₂ needed to prepare a solution, you can use the formula:
![\[ \text{Number of moles} = \text{Molarity} * \text{Volume (in liters)} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/yvj06q1idfh2g37pmwj1aty6tixgieyp96.png)
Given that the molarity
 is
is
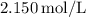 and the volume
and the volume
 is
is
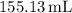 , convert the volume to liters:
, convert the volume to liters:
![\[ V = 155.13 \, \text{mL} * \left(\frac{1 \, \text{L}}{1000 \, \text{mL}}\right) = 0.15513 \, \text{L} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/5bbn4x14d8m9hg21k4igaf287bj26vqvtn.png)
Now, calculate the number of moles:
![\[ \text{Number of moles} = 2.150 \, \text{mol/L} * 0.15513 \, \text{L} = 0.3330225 \, \text{mol} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/m8bd0rhkkjgj35kplhemyhv7kjo8ik627z.png)
Next, use the molar mass of Ni₃(PO₄)₂ to find the mass:
![\[ \text{Mass} = \text{Number of moles} * \text{Molar mass} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/u2u5tvnvsoo7zco6hiptwsppwo9ml4ljny.png)
The molar mass of Ni₃(PO₄)₂ is calculated in a previous response as
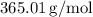 .
.
![\[ \text{Mass} = 0.3330225 \, \text{mol} * 365.01 \, \text{g/mol} = 121.68 \, \text{g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/jy70g97xyi2jpmczswh0gom12vi1ze47ny.png)
The closest answer among the choices is
 (Option C). Therefore, the correct answer is:
(Option C). Therefore, the correct answer is:
![\[ \text{C. 119.23 g} \]](https://img.qammunity.org/2024/formulas/chemistry/high-school/8q0y55jsn5o86glcu91p44mmi8mnlaao7s.png) .
.